Notes:
Old location: http://burtonsys.com/climate/sea_ice/
New location: https://sealevel.info/climate/sea_ice/
Start with one gallon of chilled tapwater:
Notes:
Zero the scale with a container on it:
Seawater contains about 35 g/L of salt, mostly NaCl.
1 Gal = 3.7854 L * 35 g/L = 132.5 g = 4.67 oz
So measure 4.7 oz of table salt:
Stir until the salt is dissolved:
Weigh an 18.0 oz chunk of ice (frozen tapwater):

(Scale's LCD display, lightened for better readability.)
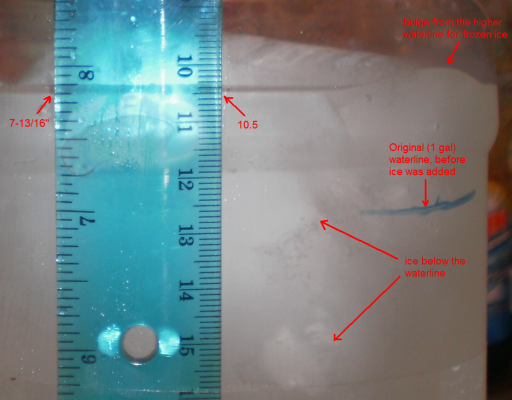
Tape a ruler to the container, and put the ice in. Note the waterline, at the 10.5 cm mark:
Another view of the floating ice:
Seal the container with plastic wrap, to prevent evaporation/condensation:
Closeup: waterline is at the 10.5 cm mark (or 7-13/16" on the other side of the ruler):
Zooming in on the same photograph:
(The photos on this page are shrunken versions; click on the photo to see the full-sized version.)
Looking down on the ice from above:
About half-melted. Water still at the 10.5 cm mark:
Note: I wiped away some of the condensation with my finger so you could see the waterline clearly.
(The upward bulge in the waterline to the right is where the ice has floated over to the side of the container.)
All but about a thimble-full of the ice has melted, two views. Water still at the 10.5 cm mark:
(I wiped away the condensation on the front with a cloth.)
A few hours later, the water is close to room temperature (two views). Waterline still at the 10.5 cm mark:
When floating ice melts in the lab it has no discernable effect on water level, unless you can make very precise measurements. In the ocean, melting sea ice has no significant effect on coastal sea levels at all.
The photos on this NSIDC web page, credited to "Professor Kay R. Brower,
of the New Mexico Institute of Technology, Socorro," appear to be a hoax.
Eyeballing the two photos, it appears that roughly the entire volume of the
ice above the waterline has added to the water level, which would be impossible
even with a fully-saturated salt solution. I suspect that the ice in her
photo is resting on the bottom of the beaker (invalidating the result):
http://nsidc.org/news/press/20050801_floatingice.html
or http://www.webcitation.org/62My1ldmN

(Yet this error got through peer review and was published!)
When floating ice melts in the lab, there is one secondary effect which very slightly affects water level: salinity. The mixing of fresh (or nearly fresh) meltwater with sea water reduces its salinity, which reduces its density. The effect is too small to see in my experiment (less than 1 mm of water level change), and not directly applicable to melting sea ice in the ocean.
When meltwater from sea ice reduces the salinity of an area of the top layer of the ocean, the "excess" volume of meltwater (the volume beyond that of an equal mass of sea water), or of mixed melt and seawater, rises up, in place, creating a locally elevated sea level, but having no effect at all on sea levels beyond the area of ocean to which the meltwater actually flows/mixes.
In a beaker, the meltwater immediately flows throughout & mixes with the whole body of water. But that is not the case in the open ocean. There the lower density liquid water rises up in place, just as lower-density solid (frozen) water does.
It doesn't matter whether the water is solid, liquid or slush, nor whether it contains voids/bubbles. Its displacement is the same because its mass is the same, and as long as its density does not exceed the surrounding seawater (which it never will) its varying density has no effect whatsoever on sea level elsewhere.
Now, when the water is liquid, it gradually mixes with the surrounding ocean, and eventually (by which I mean hundreds of years) the lower density & salinity sea water which results from mixing with meltwater will find its way into the ocean depths. A reduction of density there will affect sea levels everywhere (to a miniscule extent). But density changes in water at the surface have no effect at all on sea level elsewhere.
Not just immeasurably small, but truly zero.
Now, if you do the experiment in a beaker, in the lab, there are important differences from the real ocean. Since the container is small, It takes very little time for the meltwater to mix with and reduce the salinity throughout the beaker. The small size of the beaker means that there's really no such thing as locally elevated sea level unless some of the water is frozen solid; when the water is liquid, it quickly mixes throughout (unlike in the ocean). However, the effect on water density & depth of the change in salinity is still very small.
From my experiment you can see that, even in the lab, an amount of ice sufficient to raise the water level by 22 mm when added to the container caused only an imperceptibly small additional increase in the water level by lowering salinity when the ice melted. The effect on water level of adding a quantity of liquid water or (previously-grounded) ice is more than 30 times greater than that of melting the same quantity of already-floating ice.
In the ocean, because the reduced salinity from melting sea ice is a local effect, melting sea ice does not affect coastal sea levels at all.
Note: Click on any picture on this web page to view a larger version.
Dave Burton
Cary, NC
http://www.burtonsys.com/email/
Oct. 11, 2011
See also:
http://www.burtonsys.com/climate/
http://www.sealevel.info/
http://wattsupwiththat.com/2011/10/10/nsf-just-now-figures-out-archimedes-buoyancy-principle/
This page:
http://www.sealevel.info/sea_ice/
http://www.burtonsys.com/climate/sea_ice/